Analysis of Aspirin by Back Titration Lab Chegg
Lab 4 - Determination of the Amount of Acid Neutralized by an Antacid Tablet Using Back Titration
Goal and Overview
Antacids are bases that react stoichiometrically with acid. The number of moles of acid that can be neutralized by a single tablet of a commercial antacid will be determined by back titration. To do the experiment, an antacid tablet will be dissolved in a known excess amount of acid. The resulting solution will be acidic because the tablet did not provide enough moles of base to completely neutralize the acid. The solution will be titrated with base of known concentration to determine the amount of acid not neutralized by the tablet. To find the number of moles of acid neutralized by the tablet, the number of moles of acid neutralized in the titration is subtracted from the moles of acid in the initial solution.
Objectives and Science Skills
-
•
Understand and explain standardization as it applies to acidic and basic solutions used as reagents in an experiment. -
•
Define back titration and explain why it is used. -
•
Determine the average acid neutralizing capacity of an antacid and its associated standard deviation based on statistical treatment of data from multiple titration trials. -
•
Quantitatively and qualitatively compare experimental results with theoretical values and evaluate factors that may contribute to observed deviations.
Suggested Review and External Reading
-
•
data analysis and reference material; relevant textbook information on acids and bases
Background
Acid-base reactions and the acidity (or basicity) of solutions are extremely important in a number of different contexts — industrial, environmental, biological, etc. The quantitative analysis of acidic or basic solutions can be performed by titration. In a titration, one solution of known concentration is used to determine the concentration of another solution by monitoring their reaction. Recall that concentration is often reported in molarity, M. For example, a 1.019 M HCl solution means 1.019 moles of HCl have been dissolved in 1 L solution. A common way of representing molarity is to write 1.019 mol/L HCl, or [HCl] = 1.019 M. Also recall that molarity is a conversion factor between moles and volumes of solutions. ( 2 ) moles = ( 3 ) H+(aq) + OH–(aq) → H2O(l) ( 4 ) Mg(OH)2 + 2 HCl ( 5 ) tablet[Mg(OH)2/CaCO3] + HCl → neutralized tablet + excess acid → acidic solution ( 6 ) V H+ × M H+ = n H+ = n OH– = V OH– × M OH– ( 7 ) [OH–])
2 H+(aq) + CO3 2–(aq) → H2O(l) + CO2(g) Mg2+ + 2 Cl– + 2 H2O
Mg2+ + 2 Cl– + 2 H2O
CaCO3 + 2 HCl  Ca2+ + 2 Cl– + CO2(g) + H2O
Ca2+ + 2 Cl– + CO2(g) + H2O
excess HCl + NaOH → neutral solution
orn H+ = V OH– [OH–]
n HCl total = n HCl neutralized by tablet + n HCl neutralized by NaOH (V HCl × M HCl) = (n HCl neutralized by tablet) + (V OH– × M OH– ) or (n HCl neutralized by tablet) = (V HCl × M HCl) – (V OH– × M OH– )  H+(aq) + In–. HIn is the undissociated form that is dominant at lower pH levels; In– is the conjugate base (remains after dissociation) that is dominant at higher pH levels. HIn has one color and In– another. The equilibrium constant for this weak acid is: The pH of the solution changes by about 4 pH units around the equivalence point. This means that [H+] (and
H+(aq) + In–. HIn is the undissociated form that is dominant at lower pH levels; In– is the conjugate base (remains after dissociation) that is dominant at higher pH levels. HIn has one color and In– another. The equilibrium constant for this weak acid is: The pH of the solution changes by about 4 pH units around the equivalence point. This means that [H+] (and
Procedure
1
Follow the procedure outlined for buret usage. Be sure your buret is clean and the stopcocks are firmly seated.
For practice:
-
1
Put some water in the buret and practice controlling the stopcock. Do not fill burets on the work-bench. Always keep all chemicals below eye level. This decreases the chance of getting chemicals in your eye in the event of a spill. -
2
If you have air bubbles in the buret, gently knock the bottom of the buret to free them so they can rise to the surface. -
3
You will determine the volume of titrant delivered by subtracting the initial buret reading from the final (volume by difference). -
4
Mount the buret on the stand. In real titrations, you would put a white towel or piece of paper over the dark base of the ring stand so the color change of the indicator will be easy to see. Since this is a practice, your titrant is water. You're just practicing the stopcock control and volume reading. The goal is to get a feel for the buret. -
5
Practice reading the volume (liquid level at the bottom of the meniscus). Take readings to 0.01 or 0.02 mL. -
6
Record the initial volume of water. Add water to a collection flask and read the new volume. Find the volume of water added by difference. -
7
Practice by delivering a milliliter, a few drops, and one drop.
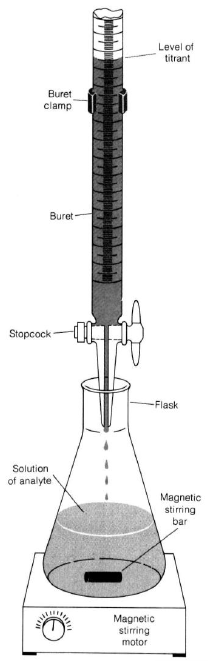
Figure 1
2
Set up a 50-mL buret with the stock NaOH. It may help you to start with Part 3 because it takes some time for the solution to heat up and cool.
Part 1: Standardization of NaOH (if necessary)
Determine the concentration of the base, NaOH, by titrating a known mass of the monoprotic acid, KHP, to neutral (the equivalence point). The molar mass of KHP is 204.23 g/mol, and it has one acidic hydrogen per molecule.
1
Precisely weigh out approximately 1.000 g potassium acid phthalate (KHP). About 10 mL of NaOH should be used in the titrations. The NaOH solution's concentration is about 0.5 M. The molar mass of KHP is 204.23 g/mol, and it has one acidic hydrogen per molecule.
2
Put the KHP into 50–100 mL water in a 250-mL titrating flask. It does not need to dissolve completely, and you don't need to know how much water is in the flask. The KHP is functioning as a strong acid and will dissolve as it is titrated. You can warm the water to aid the dissolution if needed.
3
Use a few drops of BTB as indicator in the titration flask.
4
Record the initial volume of NaOH from the buret and then begin the titration. As you turn the stopcock, push it into the barrel so it doesn't loosen and leak.
5
Record the color change at the end point and the final volume on the buret. The volume of NaOH used = V final – V initial.
6
Perform three titrations with the NaOH to obtain reproducible results.
Part 2: Standardization of HCl (if necessary)
To determine the precise molarity of the HCl solution, titrate it with the NaOH to the endpoint; use BTB as the indicator unless instructed otherwise.
1
Use a volumetric pipet to transfer exactly 10 mL of stock HCl into a 125 mL Erlenmeyer flask.
2
Record the initial volume of NaOH and titrate the HCl.
3
Record the color change at the end point and the final volume of NaOH. The volume of NaOH used = V final – V initial.
4
Repeat to be sure you can get reproducible results.
STOP — if you were not instructed to do parts 1 and 2, record the molarities of the HCl and the NaOH in your notebook. The molarities values listed on the bottles are to the ten-thousandth place (four decimal places).
Part 3: Determination of the Amount of Acid Neutralized by an Antacid Tablet
You will first react the antacid tablet with a known amount (volume) of the standardized HCl. Then you will titrate the remaining HCl with the standardized NaOH to determine the amount of acid that was not consumed by the antacid tablet. Please make sure that you have recorded the molarities of the NaOH and HCl (on the reagent bottles to four decimal places).
1
Rinse all the glassware you will be using. You must have data for at least four good trials. Please make sure you follow your TA's instructions carefully.
2
Record the mass of four antacid tablets to the nearest 0.01 g (pan balance). Each tablet will weigh a different amount, so keep track of which tablet is in which flask (see step 3).
3
Label four 125 mL Erlenmeyer flasks. To each flask add about 25 mL of distilled water.
4
Using a volumetric pipet, accurately add 25 mL of HCl and an antacid tablet. Make sure to record the molarity from the bottle if you did not standardize it. The 25-mL volumetric pipet has an uncertainity of ±0.03 mL.
5
Heat gently to a near boil for about 5 minutes, carefully avoiding splattering.
6
Be sure that the tablets are completely dissolved before titrating the solutions.
7
Allow the solutions to cool (to touch).
8
Add a few drops of BTB indicator.
9
Record the molarity of the NaOH (if you did not standardize it). The first titration may be a trial to learn approximately what volume of NaOH is needed to reach the endpoint and to become familiar with the color change at the endpoint.
10
Record the initial volume of NaOH to 0.01 mL.
11
Add NaOH in about 1 mL portions while swirling the solution. Stop between additions to swirl for a moment and observe the color. When you begin to see temporary faint color changes, add the NaOH in 0.5-mL increments. Near the endpoint, add the NaOH dropwise.
12
Record the final volume on the buret to 0.05 mL when you reach the endpoint. Save the solution in the flask as a reminder of the final color. The volume of NaOH required is V final – V initial; report the volume needed to 0.05 mL.
13
Accurately titrate the three remaining samples.
14
Dispose of your waste solutions in the waste containers in the back hood. Clean your bench top and rinse your glassware. Return any equipment that you borrowed (clean).
15
Calculate the number of moles of HCl, n H+ , to four sigificant figures using the volume and molarity of the HCl solution. This is the total amount of acid requiring neutralization (by the tablet and the NaOH).
16
Calculate the number of moles of NaOH titrant that you added to four significant figures using molarity and volume. This is the number of moles of HCl neutralized by the NaOH.
17
Determine the number of moles of HCl not neutralized by the NaOH to four significant figures. This is the number of moles of HCl neutralized by the antacid.
( 9 )
n acid neutralized by tablet = n acid initially in flask – n acid neutralized by NaOH
18
Find the average number of moles of HCl neutralized by the tablet and standard deviation.
19
Compare the average with the amount theoretically expected based on the label. Express this comparison as the % ratio of the actual amount of acid that a tablet neutralizes to the theoretical amount that it should neutralize (to three significant figures).
( 10 )
% = 100% × (n acid actually neutralized) / (n acid theoretically neutralized)
This could be less than 100% if the tablet does neutralize as much as expected or more than 100% if it exceeds what is claimed on the label.
20
Use the average moles of HCl neutralized by the tablets and the average mass of the tablets to determine the moles of acid neutralized per gram of tablet (to three significant figures). This is a more universal neutralization expression (it is independent on the mass of the tablet).
Reporting Results
Complete your lab summary or write a report (as instructed).
Results
Part 1. M OH– individually and average (with error). If you did not do this step, please write the molarity of the NaOH. Part 2. M H+ individually and average (with error). If you did not do this step, please write the molarity of the HCl. Part 3. Antacid results
Discussion/Conclusions
- What you did, how you did it and what you determined
- What were possible experimental reasons for error (deviations from expected values)
- How consistent were your tablets in the amount of antacid they contained?
Review
Analysis of Aspirin by Back Titration Lab Chegg
Source: https://www.webassign.net/labsgraceperiod/ucscgencheml1/lab_4/manual.html
0 Response to "Analysis of Aspirin by Back Titration Lab Chegg"
Enregistrer un commentaire